Pollution: the presence in or introduction into the environment of a substance which has harmfull or poisenous effects.
Point Source Pollution
Point source pollution refers to incidences of pollution that can be traced to a single location on a map. The nature of it means that the spread of the pollution can be be tracked. The causes of point source pollution are generally relatively easy to identify due to the ability to trace the source of pollution. This enables authorities to gather evidence and take action against companies/individuals that pollute the environment.
Typical examples include
- chemicals being discharged into a river by a specific factory
- toxic gases being discharged into the atmosphere by a factory.
BP Deepwater Horizon Oil Spill, 2010
In 2010 an oil rig in the Gulf of Mexico exploded and sank. The oil that it had been extracting from under the sea floor began gushing into the ocean. Stopping the oil leak posed many difficulties due to the depth at which the rig weas working and the oil spilled out for almost 3 months. Estimates put the amount of oil leaked at almost 5 million barrels. The oil plume has had significant impacts on the marine and coastal environments with BP still engaged in a huge clean up operation.
Due the point source nature of the accident authorities and agencies have been able to monitor the spread of the oil, identify some of the impacts and take action against BP. To date they have paid out over $40 billion of fines and compensation.
Union Carbide Chemical Spill, Bhopal 1984
The disaster at Bhopal was the world’s worst ever industrial accident. Exact numbers are unknown, but about 8,000 people died from poisoning within 72 hours of 40 tons of highly poisonous methyl isocyanate (MIC) pouring into the sky. A further 15,000 people had died in the years afterwards as a direct result of long-term gas-related effects, with 100,000 people continuing to suffer from chronic and debilitating illnesses for which treatment is largely ineffective.
Non-Point Source Pollution
The release of pollutants from numerous, widely dispersed origins; for example, gases from the exhaust systems of vehicles.
Non-point source pollution poses challenges for management since it difficult to identify the polluters and to impose penalties for polluting. Atmospheric pollution is a good example of the "tradegy of the commons" which is the concept that pollution of shared resources such as the atmopshere impact negatively on everyone. The polluters often gain through the reduced cost of managing or reducing the pollution and the cost is passed onto everyone else in the form of lower air quality and asociated health and environmental impacts.
The oceans, rivers and common land are other examples of shared resources that suffer the same consequences as the atmosphere.
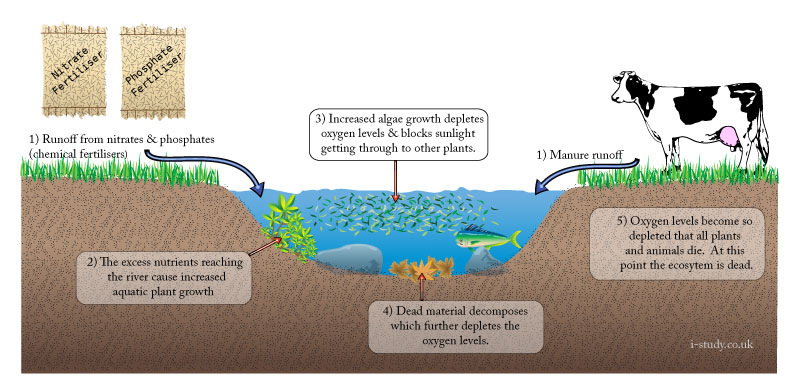
Eutrophication
Increases in the levels of nutrients entering a river/freshwater system can lead to eutrophication. Whilst it may occur naturally, modern agricultural techniques have caused many instances of it. At its most severe, eutrophication can lead to the death of a river ecosystem through de-oxygenation and lack of sunlight.
Causes
Agriculture: large scale increases in the use of chemical fertilisers containing nitrates and phosphates to increase output have resulted in increased levels of these chemical leaching into rivers or being washed in as surface runoff.
Urban areas: polluted water overflows from storm drains and sewers in times of heavy rain, these add nurtients into the river.
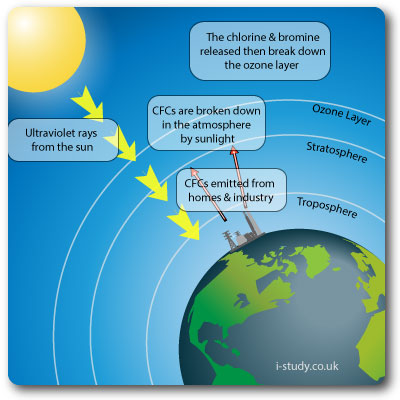
Ozone Layer Depletion
The ozone layer provides important protection from UV radiation. Without the ozone layer life as we know it would not exist.
CFCs were widely used in refrigeration appliances & aerosols (as propellants). They breakdown in the atmopshere into chlorine and bromine. The chlorine then destroys the ozone.
Depleting the ozone layer lets more of the damaging UV rays through the atmophere which negatively affects plants and humans (increased rates of skin cancer).
CFCs have largely been replaced with HCFCs, which are much less damaging to the ozone layer.
Ozone in this layer is considered "good ozone"
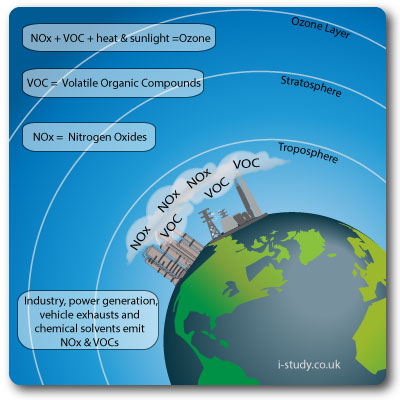
Tropospheric Ozone Pollution
When fossil fuels are burned, two of the pollutants emitted are hydrocarbons (from unburned fuel) and nitrogen monoxide (nitric oxide, NO). Nitrogen monoxide reacts with
oxygen to form nitrogen dioxide (NO2), a brown gas that contributes to urban haze. Nitrogen dioxide can also absorb sunlight and break up to release oxygen atoms that combine with oxygen in the air to form ozone.
Ozone is a toxic gas and an oxidizing agent. It damages crops and forests, irritates eyes, can cause breathing difficulties in humans and may increase susceptibility to infection. It is highly reactive and can attack fabrics and rubber materials.
Ozone in this layer is considered "bad ozone"
Management Strategies for Urban Air Pollution
London: United Kingdom
London repeatedly fails to meet European air quality standards. It is focused on reducing the emissions from vehicles as a major step towards decreasing atmospheric pollution. Some of the strategies it has implemented are:
- Congestion charge: vehicles must pay 11.50 per day to enter the central London area between 7.00am and 6.00pm Monday to Friday. It has reduced the number of cars in the congestion zone and the money generated pays for the enforcement of the system and also goes towards improving public transport.
- Bus Fleet upgrades: The bus fleet for London is in the process of being upgraded to much lighter and more fuel efficient hybrid vehicles.
- Taxis: these are expected to have zero emission capability by 2018.

Industry and Manufacturing
Stricter rules and regulation of atmospheric pollution has played a role in the decrease in heavy manufacturing around many European cities.
Polluting industries have either shutdown or relocated to other regions of the world, often developing countries. Steel manufacturing is a good example.
Fossil fuel based power stations are located outside urban areas to reduce the impact of their emissions on city centre areas. Battersea power station (London) is an example of an old power station that is being redevloped as luxury flats.
Transport
Increasing the efficiency of petrol and diesel cars has been a major focus of the automotive industry over the last few decades. Reducing the cost for the owners and producing less pollution have been the main incentives.
In recent years hybrid technologies have been adopted in many different car brands and fully electric cars are increasingly being used in urban areas. The ability to run on electricity in urban areas on short journeys but then switch to petrol/diesel on motorways allows owners to reduce emissions in cities and retain performance/speed on the highways.
This change in the efficiency and fuel type of private transport has the potential to significantly improve air quality in urban areas.
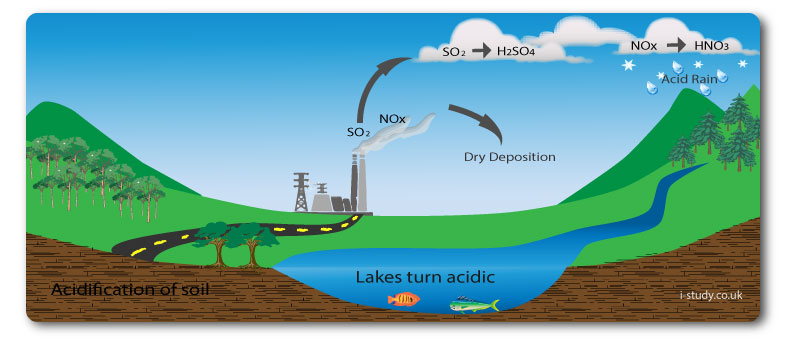
Acid Deposition
Acid deposition is the term for acid come to the earths suface from the atmosphere. It comes down in two main forms. Dry deposition is when it comes down as gas or dry particles. Wet deposition is when it comes down as part of precipitation.
Causes
The main cause of acid deposition is the burning of fossil fuels by transport and industry. This releases sulphur dioxide and nitrogen oxides into the air. These can mix with water vapour becoming sulphuric acid and nitric acid which fall to earth as acid rain.
Effects
Forests: acid rain significantly damages coniferous forests and ins some cases kills the trees. The acid rain damages the leaves/spines reducing the capacity for photosynthesis. Acidification of the oil damages the roots reducing nutrient uptake. These combined effects weaken the trees.
Rivers & lakes: freshwater bodies become increasingly acidic which harms fish and other aquatic organisms. Scandinavian lakes have been significantly impacted by acid rain. The increase in acidity caused a significant decline in the fish levels.
Leaching: acid rain also increases the leaching of aluminium, lead and mercury from soils into rivers which are particularly harmfull.
Soils: increased acidity of soils leads to increasing leaching of nutrients which leaves them less fertile. Many plants cannot tolerate acidic soils and are subsequently harmed, possibly to the death.



