The Energy Balance
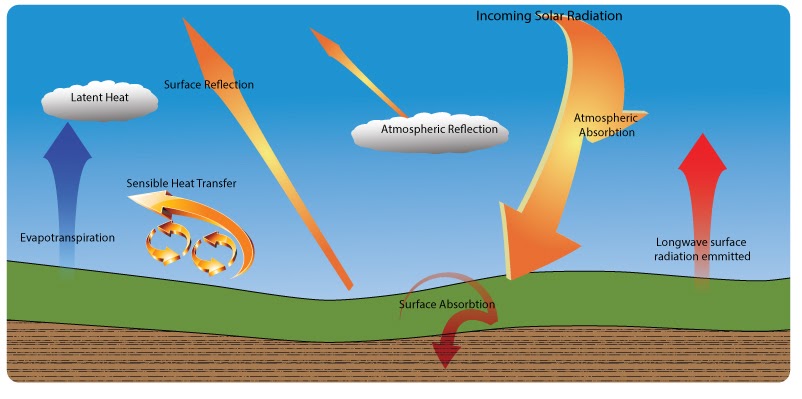
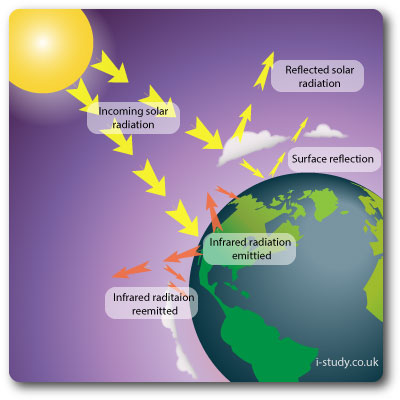
The earths atmosphere is an open system, receiving energy from both the sun and the earth. Energy is also lost out out to space. Sensible heat transfer is heat energy transferred between the surface and air when there is a difference in temperature between them. Latent heat transfer is the energy used to cause a transformation from water to water vapour (evaporation).
Tasks
- Describe in your own words the factors that maintain a general equilibrium in atmospheric temperatures.
- Research and define what the albedo rate is?
- Describe how changes in the albedo rate will affect the energy balance.
- Describe how changes in the amount of cloud might affect the energy balance